
Product Launches in the Pharmaceutical Industry
Project Support and Implementation Using SharePoint Technologies
Product launches made easy
A product launch is always a project of high complexity with numerous internal and external interfaces. This is particularly true for the pharmaceutical industry, as in addition to general market launch steps such as marketing campaigns and pricing measures, strict requirements regarding regulatory compliance must also be met.
We, the experts at msg industry advisors, have developed a tool-supported and pre-configured product launch solution in collaboration with partners in the pharmaceutical industry. This solution allows our clients to focus on and precisely monitor relevant aspects of such projects.
Product Launches for Original Drugs
The lengthy process from patenting to the actual product launch takes an average of 13.5 years, as shown in the graph below. Given that the patent term is 20 years, only 6.5 years of remaining patent protection are available for marketing an original pharmaceutical product, during which the costs for product development, approval, and tied capital, among others, must largely be recouped.
Pharmaceutical product launches are often very expensive and risky, as only one in 10,000 examined substances typically reaches market maturity. Extending the product marketing period is therefore a key success factor in the pharmaceutical industry. This extension creates a larger time window to recoup total costs of up to €1.6 billion (IFPMA 2012).
Product Launches for Generics
However, launching a generic drug is no less complex, as achieving maximum market share requires ensuring optimal market supply on the day the patent expires. The complex approval process for the medications presents an additional challenge. In Europe, approval requirements are relatively uniform. However, countries in Asia, America, and Switzerland require local approvals with significant differences in data requirements. To successfully address these challenges, competition for the generic market share often begins shortly after the original product is launched.
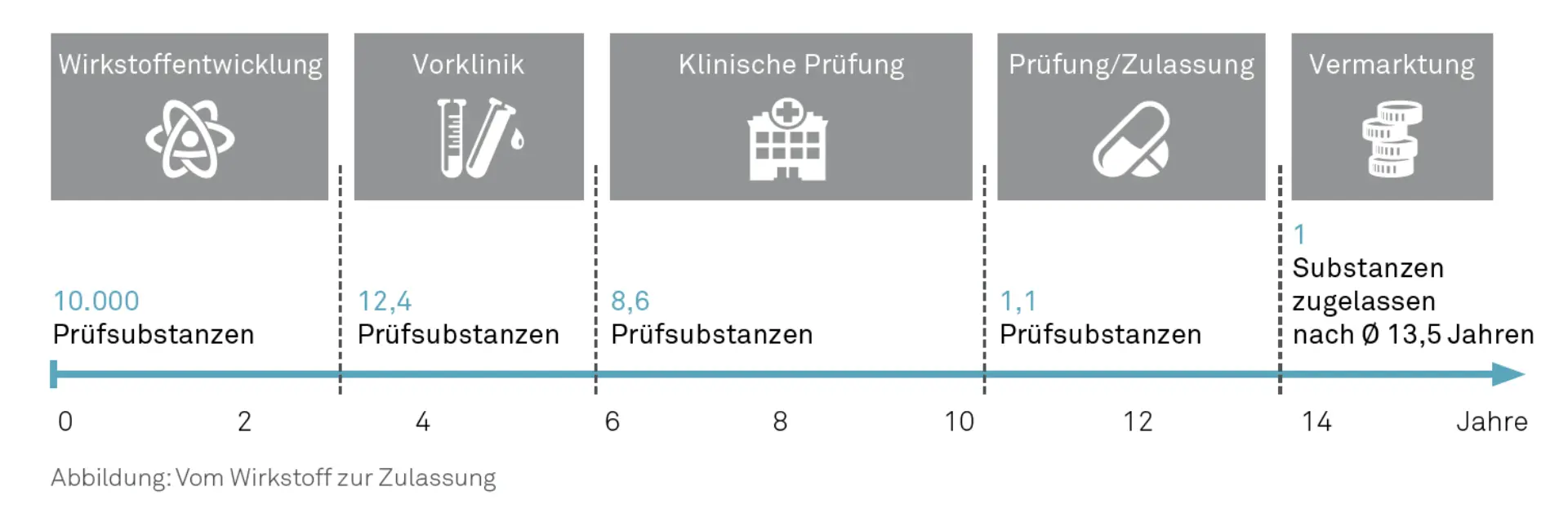
Grafik: Weg vom Wirkstoff zur Zulassung © msg industry advisors ag
Challenges
For companies, this raises the question of which tools can effectively and efficiently support such complex launch projects. It is important for project members to receive all relevant information, as only then can the necessary results for success be achieved. Additionally, project progress and performance must be measurable using jointly agreed-upon indicators.
Solution
We, the experts at msg industry advisors, have developed a tool-supported and pre-configured product launch solution in collaboration with partners in the pharmaceutical industry. This solution allows our clients to focus on and precisely monitor relevant aspects of such projects.
Our solution can support and ease your workload in managing the following tasks in the launch process:
The solution enables comprehensive project planning and management (e.g., schedules, resources, responsibilities) and evaluation (earned value analysis, milestone trend analysis, etc.) according to internationally recognized best practices of the PMI methodology.
The solution includes a set of general project KPIs, such as budget adherence/planning, on-time delivery, and team development, as well as launch process-specific KPIs, such as the number of advisory boards conducted and the training level of field staff. All KPIs can be customized to meet the specific needs of the supported project.
To optimally support your project, the solution offers a range of customizable and fully configurable templates from all areas of project management (e.g., templates for meeting minutes, earned value analyses, issue and risk logs). Of course, you can also use your own templates and documents.
Key milestones and deliverables on the critical path can be summarized into so-called launch readiness assessments to ensure the progress within the project and organization remains transparent at all times.
The solution includes mechanisms and workflows to optimally support and monitor deliverables such as marketing materials or budget reports. For example, responsible parties can be reminded of upcoming deadlines and tasks, and completed deliverables can be distributed to authorized readers based on profiles. Digital signatures or approvals enable paperless workflows.
The solution provides a central storage location for all project documentation. Upon request, the sensitivity of certain data can be maintained through permission profiles to meet specific data protection requirements.
In addition to single projects, parallelized and semi-parallelized multi-country launches are also supported, ensuring full comparability and evaluability across project boundaries.
Summary
Our Microsoft SharePoint-based solution supports your product launch with a planning and execution system fully integrated into your existing Microsoft Office environment. It also supports the needs of mobile users on iOS, Windows, and Android systems. Furthermore, the solution includes a wealth of predefined process content (predefined KPIs, templates, basic project plans, workflows, etc.) to support your project execution. To complete our offering, the solution can also be operated as "Software as a Service" (SaaS) in our German data center upon request.
With our process expertise, proven platform, predefined and customizable content, and optional hosting, msg industry advisors offer you a comprehensive package to support your launch process. At the beginning of your project, we are happy to assist with:
- A process and architecture workshop
- A quick check of your launch process maturity
- Scoping and tailoring your personal solution
Call us and be convinced!
Author
Would you like to learn more about this topic or discuss individual challenges?
Our contact is available for a personal consultation.

