
Audit-proof Digitization
Effective Digitization as the Foundation for Greater Security During FDA Audits
The increasing number of violations identified during FDA audits highlights significant weaknesses in many life sciences companies when it comes to controlling and documenting their manufacturing processes. However, effectively minimizing compliance risks requires more than just addressing individual symptoms.
What’s needed is effective digitization – one that focuses on standardization, eliminates media disruptions, and is sustainably implemented. A harmonized MES or LIMS landscape across all departments and regions forms a crucial foundation for reliably mapping and monitoring production and laboratory processes.
The FDA issued a record number of warning letters related to Good Manufacturing Practice (GMP) in fiscal year 2024. According to the latest GMP Journal, more violations were recorded this year than in the past twenty years. Particularly affected areas include core elements of production, quality assurance, and documentation.
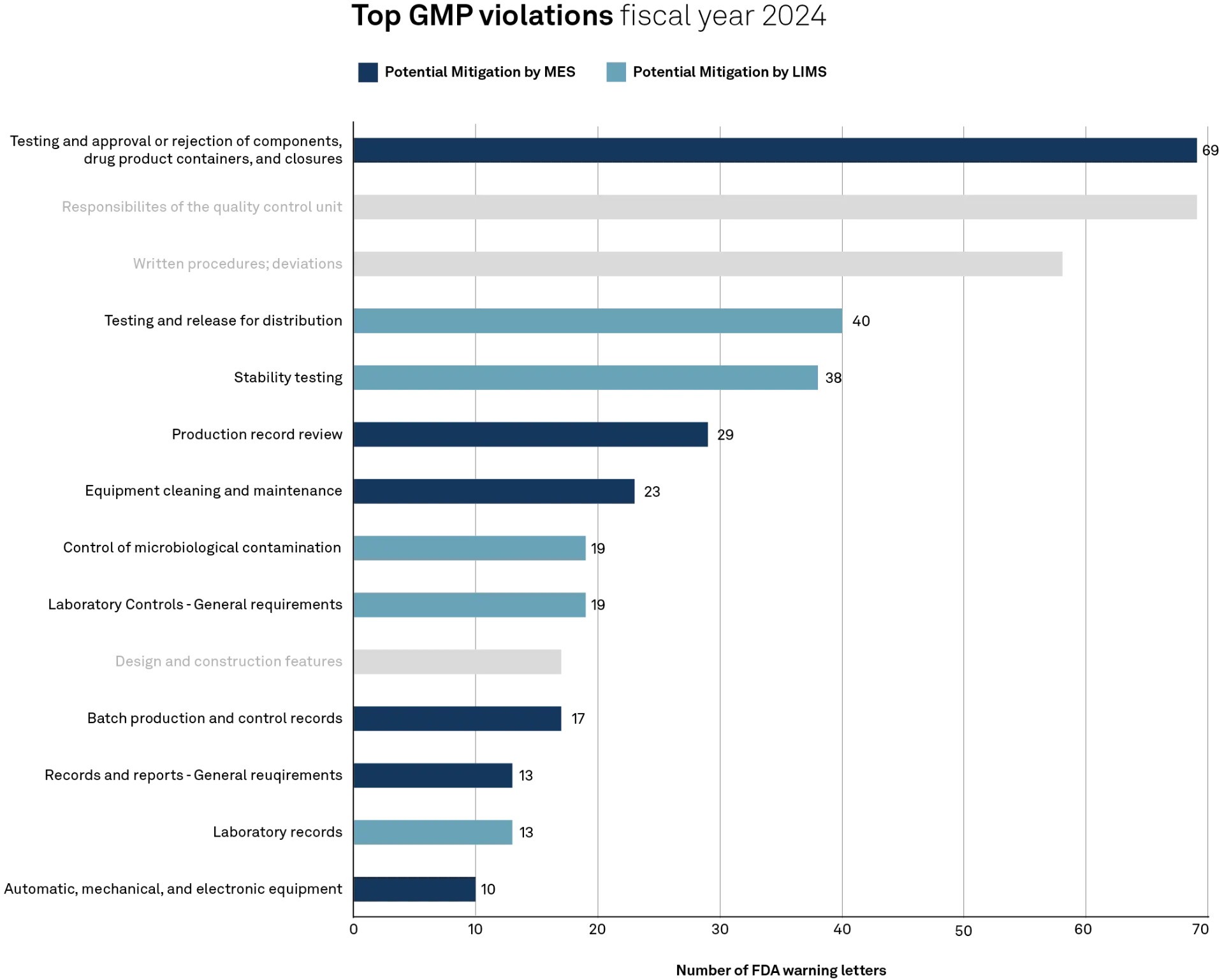
80% of the violations could have been mitigated by using an MES or LIMS
A closer look at the data reveals that most violations involve processes for the release of components and products (211.84), responsibilities in quality control (211.22), as well as deviation management and documentation (211.100).
Other significant concerns were found in the areas of stability testing (211.166) and product approval for distribution (211.165). Numerous deficiencies were also documented in laboratory practices, especially in the control of microbiological contamination (211.113) and the documentation of laboratory data (211.194).
Addressing Symptoms Is Not Enough
The analysis clearly shows that many of these violations stem from poor documentation practices and inadequate process controls. This reveals an apparent contradiction: how can violations be on the rise when the degree of digitization in life sciences production environments is also increasing?
Two key factors must be considered here. First, poor documentation is often not the root cause, but merely a symptom of deeper process-related deficiencies, such as lack of equipment qualification, insufficient maintenance, and inadequately defined or inconsistently applied procedures. Simply digitizing documentation is therefore not enough. Digitization measures must be coupled with clear process improvements, standardized procedures, and training programs to ensure sustainable compliance and to reduce deviations over the long term. Otherwise, the impact of digital systems remains limited.
Second, many of the affected companies are still in the early stages of digital transformation or are struggling with outdated, heterogeneous system landscapes. Media disruptions and lack of integration go far beyond technical issues and directly impact process reliability and compliance.
Effective Digitization as the Key to Real Process Security
The path to sustainable compliance and long-term deviation reduction is through effective digitization. Digitization is only truly effective when it maps production and laboratory processes end-to-end, is built on standardized processes across sites and organizational units and is sustainably anchored within the organization.
In many cases, the foundation of effective digitization is built on systems like centralized Manufacturing Execution Systems (MES) or Laboratory Information Management Systems (LIMS), which offer targeted opportunities to reduce risks along GMP core processes such as batch release, deviation management, and production data recording:
- MES serves as the primary tool for robust and efficient execution of production processes, ensuring optimal operator guidance and minimizing errors through digital equipment integration and real-time data validation.
- LIMS enables continuous, standardized, and compliant execution and documentation of laboratory processes. Its implementation particularly supports the fulfillment of laboratory and compliance requirements.
It’s important to note: Implementing MES and LIMS is not merely an IT project – it is a comprehensive transformation process that must involve all affected departments, from quality assurance to production. Only when processes, roles, and responsibilities are clearly defined and globally harmonized, and when employees are sufficiently empowered through training, can digitization achieve its full potential.
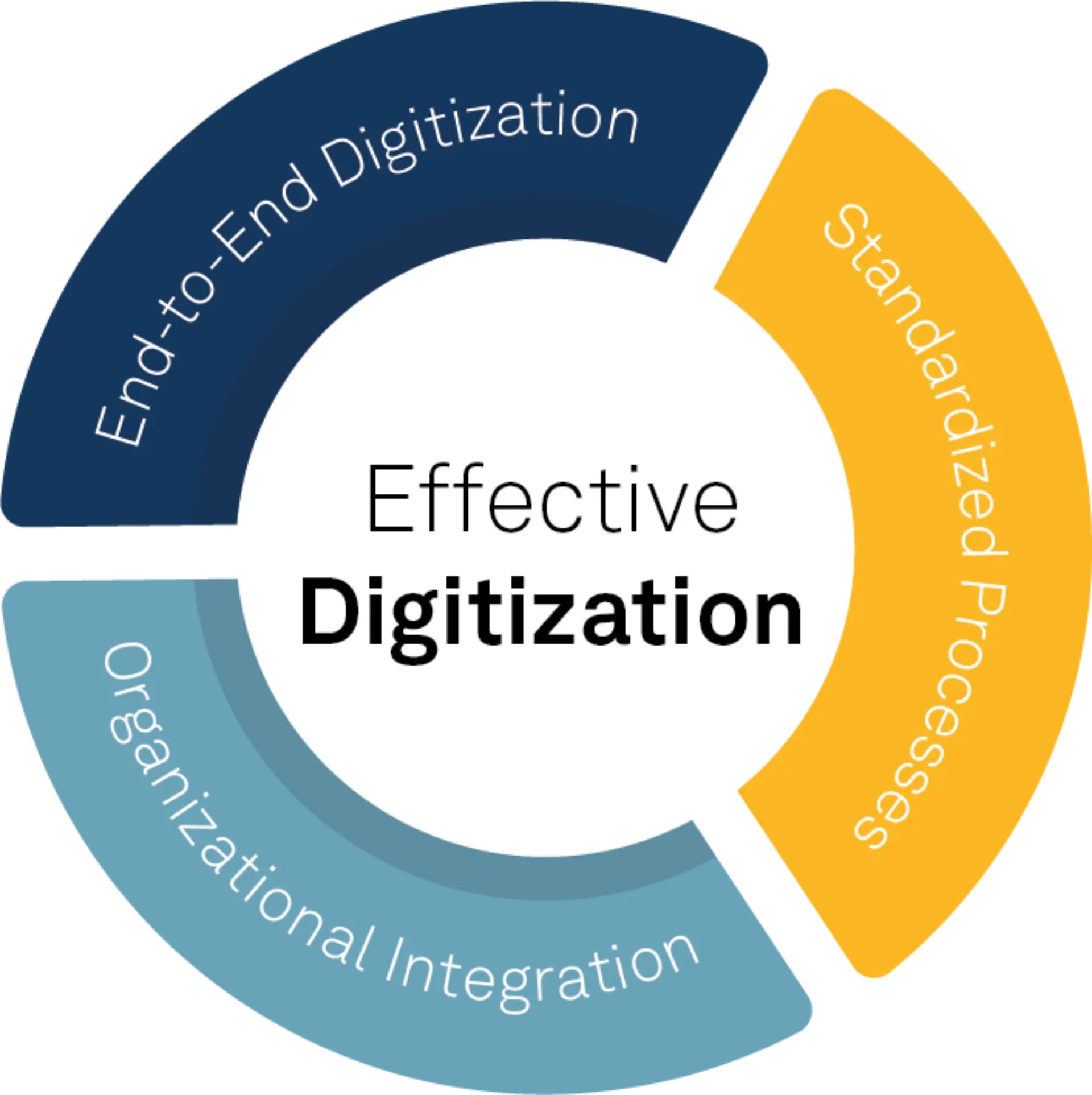
Drei zentrale Erfolgsfaktoren für wirkungsvolle Digitalisierung © msg industry advisors ag
Fast Track to Compliance: Structured, Focused, Effective
Given the record number of FDA warning letters, what can companies do in the short term to effectively reduce the risk of violations? A fast and practical route to compliant processes begins with an initial gap assessment, the use of proven templates and SOPs, and targeted training in the most affected areas.
When combined with a phased rollout strategy for MES and LIMS – such as using a Minimum Viable Product (MVP) – early successes can be achieved quickly, without forgoing the long-term benefits of full system integration. What matters most is an integrated approach combining technology, process consulting, and change management.
Professional audit preparation, including mock inspections, document reviews, and targeted training, can also help demonstrate GxP-compliant processes with confidence.
Conclusion: Digitization as a Compliance Booster
The rising number of FDA warning letters underlines the urgent need for action in the pharmaceutical and medical technology industries. Targeted digitization through MES and LIMS can reduce compliance risks and enhance process efficiency. Companies that invest in modern systems can not only meet regulatory requirements in the long term but also secure competitive advantages.
Act now: Companies that invest in MES and LIMS can avoid FDA warning letters and proactively shape the future of production and quality control.
Authors
Would you like to learn more about this topic or discuss individual challenges?
Our team is looking forward to consult you personally.


